Abstract
MDS arises through stepwise acquisitions of multiple mutations. Mutation configuration (bi-allelic vs. mono-allelic), specific distributions (hot spot), timing of acquisitions/ranks within clonal hierarchies, and combinatorial spectra, can all affect the pathogenesis and clinical phenotype of this disease. We performed integrative analyses of these processes to determine which relationships are deterministic vs. random. We analyzed 1,809 clinically annotated MDS patients. Deep targeted NGS was applied to a panel of the 36 genes frequently mutated in myeloid neoplasms. Copy number alterations (CNA) were evaluated by combining karyotyping, microarray, and digital CNA analysis. With a mean coverage of 862x, after removing SNPs and errors, we identified 3,971 somatic mutations.
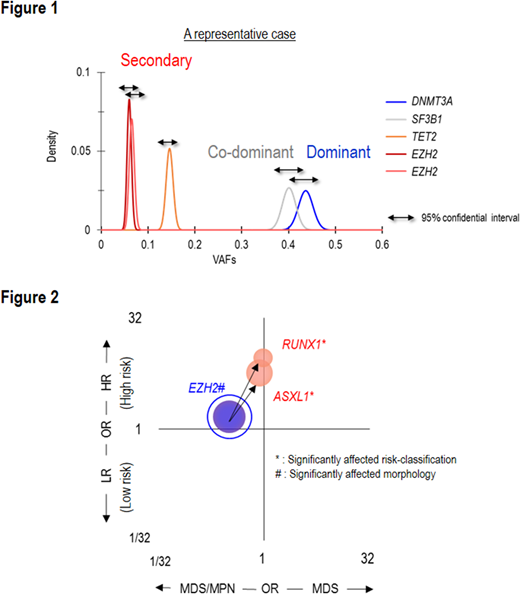
Frequently mutated genes/CNAs were TET2 (27%), SF3B1 (23%), ASXL1 (19%), del(5q) (16%), SRSF2 (14%), DNMT3A (11%) and del(7q) (10%), each present in >10% of cases. DNMT3AMT affected the canonical site (R882) in 17% (35/203) of DNMT3AMT cases, were truncating in 39% (79/203) and were other missense mutations in 44% (89/203). Bi-allelic alterations of EZH2 and TP53 most commonly involved a mutation paired with a copy number deletion or UPD. In contrast, RUNX1 and TET2 commonly involved multiple mutations of the gene. Examining intragenetic relationships, we assumed that the impact of individual mutations depends on their clonal hierarchical position. To discriminate 1st hits ("dominant") from subsequent "secondary mutations", we used a stringent binominal distribution algorithm to compare the expected vs. observed VAFs using read counts (Figure 1). A mutation with the largest VAF in a sample was defined as "dominant". Mutations with overlapping 95% CI of expected VAFs were "co-dominant", and those with non-overlapping 95% CIs were "secondary". These assumptions were validated by Pyclone (concordance rate > 95%). Accordingly, 1,474 (36%) and 1,372 (35%) mutations were dominant and secondary, respectively. SF3B1, DNMT3A, U2AF1, and TP53 were more likely to be dominant, ASXL1, CBL, ETV6, and KRAS were more likely to be secondary. For example, SF3B1 mutations were dominant in 17% (303/1809) of patients and secondary in 2% (39/1,809), p<.0001, q<.01.
78 common combinations were identified among these different types of mutations. For instance, compared to U2AF1S34 mutations, U2AF1Q157 mutations were more associated with ASXL1 mutations and del(7q), and less with DNMT3A and BCOR mutations (p<.01). Compared to TET2 mono-allelic mutations, TET2 bi-allelic mutations co-existed more with ZRSR2 and SRSF2 mutations, but less with del(5q). DNMT3AR882 mutations at 77% (27/35) were more likely to be dominant than truncating or other missense DNMT3A mutations at 51% (40/79) and 45% (47/98), p=.0046, q=.07. We also evaluated the impact of different types of mutations and their combinations on disease phenotypes and survival. Many relationships were identified between mutations in different genes and bi-allelic vs. mono allelic hits. Among frequently correlated dominant/secondary pairs, pairs of dominant EZH2 mutations and secondary RUNX1 or ASXL1 mutations were associated with higher-risk subtypes (q=.08) (Figure 2), whereas patients with dominant SF3B1 mutations and secondary JAK2 mutations had myeloproliferative features and lower-risk subtypes (q<.001; q=.09). DNMT3AR882 mutations associated with worse overall survival than did truncating and other missense DNMT3A mutations. Five pairs of dominant and secondary mutations significantly affected survival (p<.01, q<.1). We hypothesized that if a fraction of MDS originates from clonal hematopoiesis of indeterminate potential (CHIP), the compositions of dominant mutations in MDS should be similar to those in CHIP. MDS patients with dominant mutations of TET2, DNMT3A, and ASXL1 were defined as "CHIP-derived MDS" and had more secondary TET2 and ZRSR2 mutations than "De novo MDS". In conclusion, we report a comprehensive analysis of various mutational scenarios and the resultant clinical features. Most significantly, our results demonstrate how invariant patterns of evolution evolve with certain dominant hits dictating high probabilities of specific secondary events. Such patterns coincide with unique combinations of clinical properties that arise with hits along specific pathways.
Nazha:MEI: Consultancy. Sekeres:Celgene: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Opsona: Membership on an entity's Board of Directors or advisory committees; Opsona: Membership on an entity's Board of Directors or advisory committees. Haferlach:MLL Munich Leukemia Laboratory: Employment, Equity Ownership. Maciejewski:Alexion Pharmaceuticals, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Alexion Pharmaceuticals, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Ra Pharmaceuticals, Inc: Consultancy; Apellis Pharmaceuticals: Consultancy; Apellis Pharmaceuticals: Consultancy; Ra Pharmaceuticals, Inc: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal